Exploring Challenges, Successes in Solid Tumor Cell and Gene Therapy
Despite the ongoing difficulty of developing cell and gene therapies for solid tumors, some early developments have laid the foundation for future successes.
Source: Getty Images
- Cell and gene therapy advancements have significantly impacted the oncology landscape, offering an additional treatment option to patients with systemic cancers. For example, on a recent episode of Healthcare Strategies, Alivia Kaylor, MSc, senior editor at Xtelligent Healthcare Media, and Veronica Salib, associate editor, discussed the progression of cell and gene therapy, emphasizing the recent success of CASGEVY, the first FDA-approved CRISPR-based gene therapy.
Despite various successes in cell and gene therapy, the challenge remains: how can cell and gene therapies be adapted to attack solid tumors?
As the field advances, industry experts anticipate significant cell and gene therapy advancements for solid tumors.
“[The industry is] getting its hands dirty in rare diseases and blood-based cancers. Solid tumors will be another big frontier with much promise, although they are a bit more difficult to treat. Hopefully, we can make significant strides in treating these types of tumors in the next few years.” Jennifer Klarer, Head of Cell and Gene Therapy at The Dedham Group, a Norstella company, told LifeSciencesIntelligence in a 2024 interview.
Solid Tumors: Standard Treatments
According to an article in Penn Medicine, approximately 90% of all cancers are solid tumors. Johns Hopkins Medicine notes that symptoms of solid tumors may include a painless lump, pain in a specific body part, abdominal swelling, exhaustion, and unexpected weight loss.
Although the exact approach for treating solid tumors may change depending on the kind of tumor, there are a few standard approaches: surgery, chemotherapy, and radiation therapy.
In some cases, surgery can be used to eradicate a tumor. However, not all solid tumors can be entirely resolved with surgery. For more involved tumors, providers may choose to resect as much of the tumor as possible before using a secondary treatment option to kill the remaining cancer cells. Conversely, providers may choose to start with chemotherapy or radiation to shrink the tumor until it is resectable.
Chemotherapy is another standard approach for treating solid tumors; however, it is not as localized, meaning the side effects are widespread and can contribute to other challenges.
Finally, radiation therapy localizes high-energy radiation in the location of the tumor and uses it to ablate tumor cells.
Challenges in Cell and Gene Therapy for Solid Tumors
The Alliance for Cancer Gene Therapy (ACGT) notes multiple challenges associated with cell and gene therapy for solid tumors.
Heterogeneity and Hostile Tumor Microenvironments
Although CAR-T cell therapy has been successful in hematological malignancies, with several FDA approvals, the success has not translated into solid tumor malignancies. According to a 2023 article in Current Oncology Reports, solid tumors present unique cell and gene therapy challenges, including hostile tumor microenvironments (TME) and heterogeneous antigen expression.
Unlike hematological malignancies, solid tumors are more likely to have heterogeneous surface proteins, making it difficult to choose a target antigen. Additionally, the article notes that solid tumors have higher expression of tumor-associated antigens (TAA) with heterogeneity between tumor types.
These factors make designing CAR-T therapies that recognize the appropriate target antigen challenging.
“These failures highlight that effective treatment of solid tumors may depend on combination therapies, with CAR-T cells targeting multiple antigens or modified to include safety switches. Future efforts should continue to focus on combinatorial strategies to combat the immunosuppressive TME and design dosing schedules and delivery methods to enhance CAR-T cell application best. Furthermore, close attention to the safety of these therapies is especially needed in the scope of the pediatric population,” concluded researchers in Current Oncology Reports.
Toxicity
Another challenge is on-target, off-tumor toxicity. Essentially, engineered cells or gene therapies may target the appropriate protein; however, that protein may be in healthy tissues, leading to toxicity among healthy cells.
As a result, the primary challenge in treatments like CAR-T cell therapy is selecting an antigen expressed in the tumor but not in healthy tissues. Alternatively, scientists may attempt to target high expression of specific antigens. Picking a target antigen highly expressed in tumor cells but has low expression in healthy cells may reduce on-target, off-tumor toxicity.
T Cell Exhaustion
For T cell exhaustion, the organization notes that solid tumors are more resistant to therapy. Although there is no singular explanation for T cell exhaustion, ACGT postulates that protein markers may be inside of cells instead of just the cell surface and immune-suppressing TME that minimize the abilities of T cells.
“One way around this stumbling block is to focus on genes that encode proteins linked to T cell exhaustion. The National Cancer Institute reported two proteins, TOX and TOX2, as targets. Removing these two proteins from CAR T cells was one approach to diffusing exhaustion,” noted the organization’s article.
Successes in Solid Tumor Cell and Gene Therapy
Despite the challenges associated with cell and gene therapy for solid tumors, there have been some early-stage successes.
“We have seen some very encouraging results, such as the successful trafficking and infiltration into tumors and the remarkable objective responses noted in selected patients,” Samer Srour, MB ChB, assistant professor of Stem Cell Transplantation and Cellular Therapy at MD Anderson, said in an interview with MD Anderson Cancer Center. “All of this reaffirms and provides a proof of concept for the utility of CAR T cells in solid tumors.”
For example, in July 2023, the National Cancer Institute’s (NCI) Center for Cancer Research published an article on the applications of T cell therapy to address solid tumors, revealing the results of a phase 1 clinical trial.
According to the article, gavocabtagene autoleucel (gavo-cel), an experimental immunotherapy, effectively shrinks treatment-resistant solid tumors. Gavo-cell is an immunotherapy that alters a patient’s T cells to target the mesothelin protein in many solid tumors. This method is called a T cell receptor fusion construct (TRuC).
In a study published in Nature Medicine, led by Raffit Hassan, MD, Chief of the Thoracic and GI Malignancies Branch of the NCI, the researchers conducted a clinical test on gavo-cell in patients with treatment-resistant ovarian cancer or cholangiocarcinoma.
Despite the average participant having approximately five ineffective prior therapies, 40% of the participants receiving gavo-cell had a 30% or greater tumor size reduction.
“This is one of the very few adoptive cell therapies in solid tumors that have shown objective tumor regressions,” Hassan told the Center for Cancer Research. “This is proof of principle that TruC T cells work and we can get responses, even in heavily pretreated patients.”
However, nearly 80% of participants taking the medication had cytokine release syndrome, which — although it is manageable — can be fatal if poorly managed. The condition can cause symptoms, including chills, fever, fatigue, gastrointestinal issues, muscle and joint pain, headaches, edema, cognitive problems, organ failure, and death. Even so, this condition is a risk with many types of immunotherapies.
Additionally, in late 2023, an NCI study published in Clinical Cancer Research evaluated a method to enhance the efficacy of CAR T cell immunotherapy in targeting solid tumors. Researchers could target solid tumors in mouse models by engineering T cells through CAR T cells and TCR T cells to carry cytokines that boost T cell function.
The CAR T and TCR T cells in the study expressed cytokines IL-15 and IL-2 on their cell surfaces. The animal models were assigned to receive both cytokines, one cytokine, or no cytokines.
The combination approach reduced tumor size in 80% of mouse models, while only 20% of animal models treated with only one cytokine had tumor shrinkage.
As research on cell therapies for solid tumors continues to progress, it is critical to weigh the pros and cons of each type of cell therapy. Below, Putnam Inizio Advisory developed a chart that considers the strengths and weaknesses of each approach.
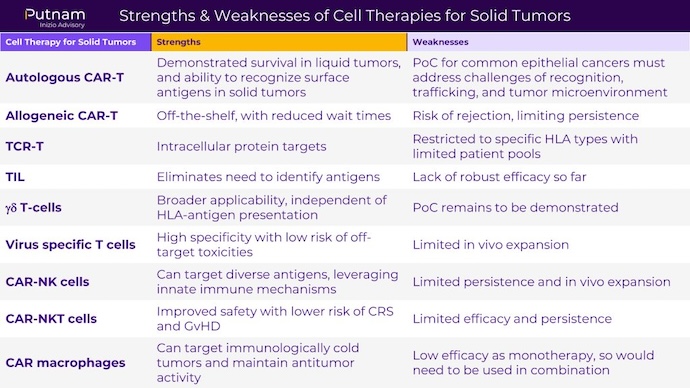
Source: Putnam Inizio Advisory
