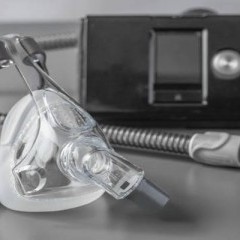
FDA Issues Update on the Philips BiPAP and CPAP Recalls
- Last week, the United States Food and Drug Administration released a statement updating consumers and industry leaders on the Philips Respironics recalls due to the potential risks associated with polyester-based polyurethane (PE-PUR) foam. The FDA Center for Devices and Radiological Health (CDRH) issued the update and attributed it to the director,...